Written by Alejandra Feliciano
What makes plants coexist?
Landscape design traditionally utilizes the fundamental niche as a basis for plant selection, without taking into account the effects of plant interactions. These interactions must be considered to effectively select plant companions that can coexist over time.
Studies in diversity-productivity relationships suggest that conventional horticultural management practices, such as management of soil fertility, irrigation, and disturbance, influence plant diversity, and that community productivity mechanisms should be managed to self-reinforce long-term diversity. Recently developed methods for functional guild detection offer tools to measure and compare species functional fitness within a plant community, which provide novel insights into weed invasion vulnerabilities and related landscape management strategies.
SUMMARY: This article will examine ecological studies and their practical application to create more diverse, stable urban landscapes. To address the topic of creating biodiverse landscapes through plant selection, I will review literature on the ecological niche as it relates to species coexistence, and on making plant selections based on coexistence theory. To address the topic of promoting biodiversity through landscape management strategies, I will review literature on the relationships between diversity and plant biomass productivity. Finally, as a practical means of addressing tolerance to weed invasion, I will examine plant community assembly and succession from a functional perspective.
Considering plant interactions when selecting landscape plants for biodiversity
German ecologist Heinz Ellenberg demonstrated the difference between the fundamental and realized niche (Ellenberg 1952). The classical definition of the niche (Grinnell 1914) describes it as the unique set of characteristics required for an organism to maintain or increase its population, but Ellenberg noted that observed species ranges do not always correspond to their expected tolerances. In some instances, species under competitive pressure exhibit more limited ranges, being absent under otherwise favorable abiotic conditions. The opposite is also possible; species are able to persist outside their expected range due to positive interactions which facilitate the expansion of their fundamental niche (Bruno et al. 2003). The realized niche, then, reflects a species’ range as limited or expanded by organismal interactions (Figure 2‑1).
Although the landscape design profession has traditionally prioritized aesthetic considerations when selecting plant companions, the concept coined by amateur plantswoman Beth Chatto “choose the right plant for the right place” has served as a rallying cry to highlight the effect abiotic considerations can have on lowering maintenance (Chatto 1978).
Nowadays most contemporary garden design publications recommend “site-adapted plant selections” (Brickell 2003, VanDerZanden and Cook 2010). Such selection criteria rely on identifying abiotic characteristics of a site, such as sunlight and temperature exposure, and matching them to plant physiological tolerances. This approach effectively addresses a plant’s fundamental niche, but it ignores the equally significant effect plant interactions have on continued persistence. The ultimate the long-term success of a planting must, then, also consider the realized niche through an understanding of plant interactions.
Understanding the requirements for coexistence
A concept intrinsic to plant community diversity is coexistence between species; the more plant species can coexist, the greater the diversity. Coexistence Theory was popularized by Dr. Peter Chesson 20 years ago (Chesson 2000a).
According to Chesson, the first requirement for stable coexistence between a species pair is persistence of both species. In other words, that there be a mechanism in place to promote the recovery species close to extinction. This is achieved through negative frequency dependence (NFD). Under NFD, intraspecific (within-species) competition limits population frequency[1], or relative abundance, more than interspecific (between-species) competition such that, as their population declines, species experience greater interspecific competition and an associated recovery in their population density. Under positive frequency dependence (PFD), growth rates and frequencies are positively correlated such that exclusion occurs at low densities (Vellend 2010). At high densities, NFD can also serve to cap growth rates, such that as a species frequency increases relative to others in the community, their per capita growth rate declines (Box 2‑1).
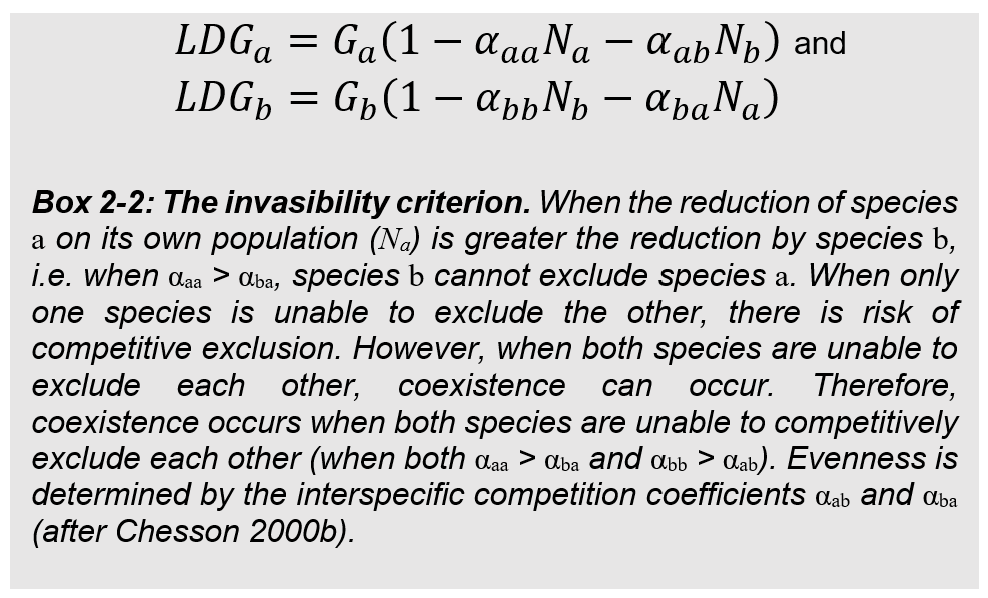
This is traditionally expressed through the invasibility criterion, a modified form of the Lotka-Volterra competition model. As shown in Box 2‑2, the invasibility criterion reveals the conditions for coexistence. To have stable coexistence, plants need to be able to recover from low density, as well as be unable to exclude each other.
The engine that powers such mutual inability to exclude is called niche partitioning. Niche partitioning means that limiting resource overlap is partially incomplete among competing species, such that it generates mutual inability to exclude. When there is partial resource overlap, the density-dependent feedback loops each species has with their respective resources make intraspecific competition greater than interspecific competition. However, when niches fully overlap, then only the inferior competitor will have inability to exclude.
This phenomenon naturally arises from trade-offs in resource use among species where, resource use and consumption vary among species due to their differing evolutionary adaptations.
This begs the question; how much niche overlap is necessary for stable coexistence? The answer varies depending on the magnitude of fitness similarity (Adler et al. 2007a). When there is enough niche difference to overcome the exclusionary effects of fitness difference, intraspecific competition is greater than interspecific competition and exclusion becomes impossible for both species (Box 2‑3).
Considering this relationship between niche difference and fitness similarity, four outcomes are possible at their extremes: exclusion, stable coexistence, unstable coexistence, or a combination of both stable and unstable coexistence (Figure 2‑2).
Figure 2-2. Coexistence as a result of niche difference and fitness similarity.
Conditions for stable and unstable coexistence
Unstable coexistence occurs when equalizing effects delay exclusion. In equalizing mechanisms, fitness differences between competing plants are small, such that they become near-equivalent, or ‘neutral’. This weakens the exclusionary effect of interspecific competition, causing competitive exclusion to be delayed. This allows plants to enter unstable coexistence for some time until stochastic factors, such as climatic changes, alter the niche and make certain populations more vulnerable to exclusion.
When there are no niche partitions, fitness differential trends towards exclusion. Fitness differential can have a sorting effect during plant interactions, whereby species with similar fitness displace each other very slowly and species with high fitness can quickly displace those with much lower fitness. When this exclusion occurs slowly, species enter unstable coexistence.
When niche overlap is low, this exclusionary process can lead to the formation of niche partitions where species are sorted by their fitness. Under these circumstances, species may coexist as long as they are ‘separated’ by the niche space in which each specializes, creating stable coexistence. Equalizing mechanisms continue to play out such that each partition continues to individually trend towards exclusion.
To summarize, when the stabilizing effects of niche partitioning surpass the exclusionary effects of fitness difference, stable coexistence can occur (Adler et al. 2007b). Therefore, stable coexistence is possible when niche differences are high regardless of fitness difference, and unstable coexistence is possible when fitness similarity is high regardless of niche differences (Figure 2‑2).
Diversity-productivity relationships: how landscape maintenance practices influence diversity promotion
Understanding the outcomes of interactions is only part of the picture when studying plant community diversity. For decades ecologists have debated the nature of relationships between productivity (the amount of biomass production in a plant community) and diversity (the richness and evenness of species). Does a relationship exist between productivity and diversity, and could it help explain diversity dynamics in different habitats?
The classic hump-backed model (HBM)
The classic theoretical model describing D-P relationships in plant communities is the hump-backed model (HBM), which was developed based on the results of two separate studies. The first study was a PhD thesis (Mahmoud 1973), which compared experimental sown grass communities subjected to nitrogen starvation (stress) and cutting (disturbance) gradients. The results showed a striking relationship between diversity, stress, and disturbance—decreased species evenness with decreasing stress, and decreased species evenness with decreasing disturbance. Although the effects of stress and disturbance on richness were similar (both positive correlations), their effect on evenness for each community varied. Highest evenness (and therefore highest plant diversity) was observed on treatments with both high nitrogen stress and frequent disturbance, not disturbance or stress alone.
The second study was an extensive survey of species richness in 2,748 samples of English herbaceous plant communities (Grime 1973a). The survey recorded many site conditions such as soil pH, soil fertility, unrestricted rooting depth, and disturbance management (grazing, burning, mowing). This field survey showed that low species evenness (strong species exclusion) occurred not just in fertile sites (low stress) associated with the dominance of the most successful competitor, it also occurred in habitats subject to high stress, high disturbance, or habitats with coinciding high stress and disturbance.
Taking a second look at the HBM in context with functional guilds. High community productivity (1) corresponds with highest fitness for competitors (C). Low productivity caused by high disturbance (3) corresponds with highest fitness for ruderals (R). When low productivity is caused by high stress (2) it corresponds with highest fitness for stress-tolerators (S). Under intermediate productivity ranges, generalists equalize investments across both the economic and size spectra such that niche difference, rather than fitness, controls diversity (4).
Based on this information, the HBM (Grime 1973b) theorized the limit of species diversity potential on a productivity gradient controlled by stress and disturbance (Figure 2‑4). Under this model, low-stress, low-disturbance environments become amenable to high biomass production, and tend to become dominated by few exclusionary competitor species, resulting in communities featuring high biomass productivity but low diversity. Stress and disturbance can promote diversity and coexistence by suppressing such dominance, but only for an intermediate range of productivities. If their effects become strong enough, disturbance and stress limit biomass production to the point where they can create communities of very low biomass productivity. At the extremes, these conditions select for highly specialized stress-tolerating and/or ruderal species, promoting their dominance and lowering diversity, resulting in communities with low productivity and low diversity (Figure 2‑4).
[2] Accessed from Google Scholar search on 7/23/2020
Five years later, other ecologists developed similar models. Al-Mufti et al. (1977) described a model very similar to the HBM, and Joseph Connell described what later came to be known as the Intermediate Disturbance Hypothesis (Connell 1978). Although arguably more popularized than the HBM (2,039 citations), the Intermediate Disturbance Hypothesis (10,255 citations) [2] could be described as an incomplete version of the HBM, in that it only considers diversity-disturbance relationships, not the relationship between diversity, productivity, stress, disturbance, and dominance.
Models other than the HBM, such as consumer-resource models, also support the prediction of greatest coexistence (and diversity) at intermediate productivities (Abrams and DeAngelis 2001). Under these models, high productivity increases the impact of apparent competition such that diversity peaks at intermediate productivities. It has been suggested that the HBM is compatible with facilitative interactions in communities on the left-hand side of the ‘hump’ and competitive interactions on its right side (Michalet et al. 2006, Xiao et al. 2009). Communities of extremely low biomass productivity lead to infrequent interactions between plants such that highly specialized plants dominate communities. As conditions begin to improve, such dominants can provide the shelter and/or nutrients necessary to facilitate range expansion for otherwise unsuitable species. When abiotic conditions become favorable enough, interactions shift from facilitation to competition such that both play a role at intermediate productivities and competitive interactions play a stronger role at the highest productivities.
Mechanisms behind the HBM
Why HBM trends are observed and the mechanisms behind them is still an active area of study. It is important to note that under the HBM, biomass productivity is not the mechanism driving diversity, instead it is a symptom of the habitat conditions of a given site. Plant biomass productivity is typically controlled by rainfall, temperature, and important soil properties such as texture, which allow for accumulation or depletion of organic matter, minerals, and other limiting resources over time.
Another important observation here is that the trends outlined by the HBM might point towards characteristics in the composition of the broader species pool. If species are excluding each other at productivity extremes, this suggests that all other things being equal, the species pool at the locations where the HBM was observed contained more species adapted to intermediate productivity regimes and fewer species adapted to productivity extremes. This hypothesis is supported by the fact that HBM curves are more common in the northern hemisphere and less common in the tropics. In tropical rainforests, where growing conditions have remained relatively stable for millennia longer than in temperate regions, high biomass productivity coincides with high diversity and therefore violates the HBM. One possible explanation for this is simply that in the tropics the species pool has had more time to select for plant species adapted to high productivity conditions, providing more opportunities for speciation and coexistence.
Applications to landscape maintenance
We have seen from the observed diversity-productivity relationships, providing favorable growing conditions may lower the chances for coexistence and diversity promotion. Horticultural practices geared toward the cultivation of edible crop species focus on increased production and yields. The most favorable growing conditions are implemented such that these crop species are afforded not only the best chances at survival, but also afforded the artificial conditions that will lead them to bear the most fecund harvest and highest crop yields. A re-examination of this approach, which has been transferred into the cultivation practices for urban ornamental landscapes, is in order. To achieve the goal of diversity promotion, landscape maintenance practices (such as management of soil fertility, irrigation, and disturbance) should be leveraged to promote the creation of communities of intermediate productivity, not high productivity.
Functional guilds and community productivity: How CSR syndromes and the converging plant responses to stress, competition, and dispersal affect diversity
Traditionally, plant communities have been described in taxonomical terms, which focus on identifying the phylogenic proportion of individual species present on a given site. More recently, scientists have found utility in describing plant communities in functional terms, which describe the functions guilds present within a community regardless of taxa.
Defining functional guilds
A framework for identifying plants based on their functional adaptations to optimize biomass production was first proposed by Dr. John Phillip Grime. This fundamental trade-off framework came to be known as the C-S-R model (Grime 1977). Through this model, plants were identified based on their proportional investment of limited resources into adaptations to produce biomass under varying abiotic stress, biotic competition, and dispersal opportunities.
A recent study sampling close to 70,000 plant species (Diaz et al. 2016), the largest sample of worldwide plant traits to date, found that plant trait space is observably constrained into pockets or ‘hot spots’ of viable trait combinations. These trait syndromes correspond with the productivity-based constraints predicted by Grime’s model. The study found that 74% of the variation in traits could be explained by two major axes, those of economics and size.
According to Grime, this is a three-way trade off, where investments in the size spectrum correspond to competitive advantage, and investments on either end of the economic spectrum represent trade-offs between stress tolerance and dispersal ability. This was previously supported by numerous studies, including the discovery of the leaf economic spectrum, which linked plant physiological responses and energy investments with their morphological traits (Shipley et al. 2006, Wright et al. 2004, Diaz et al. 2004).
To describe CSR functional guilds using these contemporary terms, the competitive guild (C) is comprised of plants with intermediate economics and large size, the stress-tolerator guild (S) is comprised of plants with conservative economics and small size, and the ruderal guild (R) is comprised of plants with acquisitive economics and small size.
How guilds are related to community productivity and diversity
The competitive guild (C) holds an advantage under low stress, low disturbance environments where dispersal and stress-tolerance ability are sacrificed for competitive ability (Figure 2‑4). This corresponds with abiotic conditions favorable for plant growth, and with plant communities of high biomass productivity at the extreme right end of the HBM. Competitors are expected to invest more heavily in chemical rather than physical defenses against stress and herbivory, which is reflected in their large leaf area, and intermediate leaf nitrogen and carbon content (Diaz et al. 2016).
The stress-tolerator guild holds an advantage under high stress, low disturbance environments, where dispersal and competitive ability are sacrificed for robust abiotic stress defense. Stress tolerators have high leaf carbon and leaf dry matter content (Pierce et al. 2013), which provides more long-term physical defenses against stress and herbivory. Stress-tolerators have carved a niche where they can dominate plant communities under abiotic conditions generally unfavorable for plant growth, at the low-productivity end of the HBM.
Similarly, the ruderal guild holds an advantage under low stress, high disturbance environments, where competitive and stress-tolerance ability is sacrificed for larger investment in dispersal ability. Ruderals have soft leaves with high nitrogen content and minimal defenses against stress and herbivory. Like stress-tolerators, ruderals have carved a niche where they can dominate in plant communities under disturbance regimes unfavorable for plant growth, at the low-productivity end of the HBM.
Under the HBM, the highest potential for diversity promotion is expected to coincide with the formation of plant communities of intermediate to low biomass production. So which guild is most successful under this target productivity range? Generalists (CSR) are species balanced in their proportional allocation of investments into all three strategies (33%-33%-33% split). I propose that species with traits associated with the generalist to ruderal (CSR/R) or generalist to stress-tolerator (CSR/S) proportions would best perform in communities of intermediate productivity in the northern hemisphere.
Such trends still need verification in the field. Until recently, efforts to observe and document functional community profiles have been hampered due to lack of agreement on a standardized method for detecting functional guilds (Hodgson et al. 1999, Pierce et al. 2013, Kattenborn et al. 2017). Most recently, a globally-calibrated method for detecting CSR functional guilds in the field was developed by scientists working on Italian alpine communities (Pierce et al. 2017). Thanks to this method, C-S-R proportions have been identified for over 3,000 species so far, mostly naturalized to Western Europe. A more comprehensive cataloguing effort is required to describe the functional composition of American plant communities.
Works Cited
Abrams, P. A., and A. E. D. L. DeAngelis. 2001. The Effect of Density‐Independent Mortality on the Coexistence of Exploitative Competitors for Renewing Resources. The American Naturalist 158:459–470.
Adler, P. B., J. HilleRisLambers, and J. M. Levine. 2007a. A niche for neutrality. Ecology Letters 10:95–104.
Adler, P. B., J. HilleRisLambers, and J. M. Levine. 2007b. A niche for neutrality. Ecology Letters 10:95–104.
Adler, P. B., E. W. Seabloom, E. T. Borer, H. Hillebrand, Y. Hautier, A. Hector, W. S. Harpole, L. R. O’Halloran, J. B. Grace, T. M. Anderson, J. D. Bakker, L. A. Biederman, C. S. Brown, Y. M. Buckley, L. B. Calabrese, C.-J. Chu, E. E. Cleland, S. L. Collins, K. L. Cottingham, M. J. Crawley, E. I. Damschen, K. F. Davies, N. M. DeCrappeo, P. A. Fay, J. Firn, P. Frater, E. I. Gasarch, D. S. Gruner, N. Hagenah, J. H. R. Lambers, H. Humphries, V. L. Jin, A. D. Kay, K. P. Kirkman, J. A. Klein, J. M. H. Knops, K. J. La Pierre, J. G. Lambrinos, W. Li, A. S. MacDougall, R. L. McCulley, B. A. Melbourne, C. E. Mitchell, J. L. Moore, J. W. Morgan, B. Mortensen, J. L. Orrock, S. M. Prober, D. A. Pyke, A. C. Risch, M. Schuetz, M. D. Smith, C. J. Stevens, L. L. Sullivan, G. Wang, P. D. Wragg, J. P. Wright, and L. H. Yang. 2011. Productivity Is a Poor Predictor of Plant Species Richness. Science 333:1750–1753.
Allcock, K. G., and D. S. Hik. 2003. What Determines Disturbance-Productivity-Diversity Relationships? The Effect of Scale, Species and Environment on Richness Patterns in an Australian Woodland. Oikos 102:173–185.
Al-Mufti, M. M., C. L. Sydes, S. B. Furness, J. P. Grime, and S. R. Band. 1977. A Quantitative Analysis of Shoot Phenology and Dominance in Herbaceous Vegetation. Journal of Ecology 65:759–791.
Bhattarai, K. R., O. R. Vetaas, and J. A. Grytnes. 2004. Relationship between Plant Species Richness and Biomass in an Arid Sub-Alpine Grassland of the Central Himalayas, Nepal. Folia Geobotanica 39:57–71.
Brickell, C. 2003. American Horticultural Society Encyclopedia of Gardening. DK.
Bruno, J. F., J. J. Stachowicz, and M. D. Bertness. 2003. Inclusion of facilitation into ecological theory. Trends in Ecology & Evolution 18:119–125.
Chatto, B. 1978. The dry garden. Sagapress, Sagaponack, N.Y.
Chesson, P. 2000a. General Theory of Competitive Coexistence in Spatially-Varying Environments. Theoretical Population Biology 58:211–237.
Chesson, P. 2000b. Mechanisms of Maintenance of Species Diversity. Annual Review of Ecology and Systematics 31:343–366.
Chow, S. S., C. O. Wilke, C. Ofria, R. E. Lenski, and C. Adami. 2004. Adaptive Radiation from Resource Competition in Digital Organisms. Science 305:84–86.
Colasanti, R. L., R. Hunt, and A. P. Askew. 2001. A self-assembling model of resource dynamics and plant growth incorporating plant functional types. Functional Ecology 15:676–687.
Connell, J. H. 1978. Diversity in Tropical Rain Forests and Coral Reefs. Science 199:1302–1310.
Diaz, S., J. G. Hodgson, K. Thompson, M. Cabido, J. H. C. Cornelissen, A. Jalili, G. Montserrat‐Martí, J. P. Grime, F. Zarrinkamar, Y. Asri, S. R. Band, S. Basconcelo, P. Castro‐Díez, G. Funes, B. Hamzehee, M. Khoshnevi, N. Pérez‐Harguindeguy, M. C. Pérez‐Rontomé, F. A. Shirvany, F. Vendramini, S. Yazdani, R. Abbas‐Azimi, A. Bogaard, S. Boustani, M. Charles, M. Dehghan, L. de Torres‐Espuny, V. Falczuk, J. Guerrero‐Campo, A. Hynd, G. Jones, E. Kowsary, F. Kazemi‐Saeed, M. Maestro‐Martínez, A. Romo‐Díez, S. Shaw, B. Siavash, P. Villar‐Salvador, and M. R. Zak. 2004. The plant traits that drive ecosystems: Evidence from three continents. Journal of Vegetation Science 15:295–304.
Diaz, S., J. Kattge, J. H. C. Cornelissen, I. J. Wright, S. Lavorel, S. Dray, B. Reu, M. Kleyer, C. Wirth, I. Colin Prentice, E. Garnier, G. Bönisch, M. Westoby, H. Poorter, P. B. Reich, A. T. Moles, J. Dickie, A. N. Gillison, A. E. Zanne, J. Chave, S. Joseph Wright, S. N. Sheremet’ev, H. Jactel, C. Baraloto, B. Cerabolini, S. Pierce, B. Shipley, D. Kirkup, F. Casanoves, J. S. Joswig, A. Günther, V. Falczuk, N. Rüger, M. D. Mahecha, and L. D. Gorné. 2016. The global spectrum of plant form and function. Nature 529:167–171.
Ellenberg, H. 1952. Physiologisches und ökologisches Verhalten derselben Pflanzenarten. Berichte der Deutschen Botanischen Gesellschaft 65:350–361.
Espinar, J. L. 2006. Sample size and the detection of a hump-shaped relationship between biomass and species richness in Mediterranean wetlands. Journal of Vegetation Science 17:227–232.
Fraser, L. H., M. Pärtel, J. Pither, A. Jentsch, M. Sternberg, and M. Zobel. 2015a. Response to Comment on “Worldwide evidence of a unimodal relationship between productivity and plant species richness.” Science 350:1177–1177.
Fraser, L. H., J. Pither, A. Jentsch, M. Sternberg, M. Zobel, D. Askarizadeh, S. Bartha, C. Beierkuhnlein, J. A. Bennett, A. Bittel, B. Boldgiv, I. I. Boldrini, E. Bork, L. Brown, M. Cabido, J. Cahill, C. N. Carlyle, G. Campetella, S. Chelli, O. Cohen, A.-M. Csergo, S. Díaz, L. Enrico, D. Ensing, A. Fidelis, J. D. Fridley, B. Foster, H. Garris, J. R. Goheen, H. A. L. Henry, M. Hohn, M. H. Jouri, J. Klironomos, K. Koorem, R. Lawrence-Lodge, R. Long, P. Manning, R. Mitchell, M. Moora, S. C. Müller, C. Nabinger, K. Naseri, G. E. Overbeck, T. M. Palmer, S. Parsons, M. Pesek, V. D. Pillar, R. M. Pringle, K. Roccaforte, A. Schmidt, Z. Shang, R. Stahlmann, G. C. Stotz, S. Sugiyama, S. Szentes, D. Thompson, R. Tungalag, S. Undrakhbold, M. van Rooyen, C. Wellstein, J. B. Wilson, and T. Zupo. 2015b. Worldwide evidence of a unimodal relationship between productivity and plant species richness. Science 349:302–305.
Grace, J. B., T. M. Anderson, M. D. Smith, E. Seabloom, S. J. Andelman, G. Meche, E. Weiher, L. K. Allain, H. Jutila, M. Sankaran, J. Knops, M. Ritchie, and M. R. Willig. 2007. Does species diversity limit productivity in natural grassland communities? Ecology Letters 10:680–689.
Grime, J. P. 1973a. Control of species density in herbaceous vegetation. Journal of environmental management:151–167.
Grime, J. P. 1973b. Competitive Exclusion in Herbaceous Vegetation. Nature 242:344–347.
Grime, J. P. 1977. Evidence for the Existence of Three Primary Strategies in Plants and Its Relevance to Ecological and Evolutionary Theory. The American Naturalist 111:1169–1194.
Grime, J. P., and S. Pierce. 2012. The Evolutionary Strategies that Shape Ecosystems. John Wiley & Sons.
Grinnell, J. 1914. An account of the mammals and birds of the lower Colorado Valley: with especial reference to the distributional problems presented. University of California Press.
Guo, Q., and W. L. Berry. 1998. Species Richness and Biomass: Dissection of the Hump-Shaped Relationships. Ecology 79:2555–2559.
Hodgson, J. G., P. J. Wilson, R. Hunt, J. P. Grime, and K. Thompson. 1999. Allocating C-S-R Plant Functional Types: A Soft Approach to a Hard Problem. Oikos 85:282–294.
Houseman, G. R., and K. L. Gross. 2006. Does Ecological Filtering across a Productivity Gradient Explain Variation in Species Pool-Richness Relationships? Oikos 115:148–154.
Kattenborn, T., F. E. Fassnacht, S. Pierce, J. Lopatin, J. P. Grime, and S. Schmidtlein. 2017. Linking plant strategies and plant traits derived by radiative transfer modelling. Journal of Vegetation Science 28:717–727.
Laanisto, L., and M. J. Hutchings. 2015. Comment on “Worldwide evidence of a unimodal relationship between productivity and plant species richness.” Science 350:1177–1177.
Laughlin, D. C., and M. M. Moore. 2009. Climate-Induced Temporal Variation in the Productivity-Diversity Relationship. Oikos 118:897–902.
Mackey, R. L., and D. J. Currie. 2001. The Diversity-Disturbance Relationship: Is It Generally Strong and Peaked? Ecology 82:3479–3492.
Mahmoud, A. 1973. A laboratory approach to ecological studies of the grasses: arrhenatherum elatius (l.) beav. ex j. and c. presl agrostis tenuis sibth. and festuca ovina l. ProQuest Dissertations Publishing.
Michalet, R., R. W. Brooker, L. A. Cavieres, Z. Kikvidze, C. J. Lortie, F. I. Pugnaire, A. Valiente‐Banuet, and R. M. Callaway. 2006. Do biotic interactions shape both sides of the humped-back model of species richness in plant communities? Ecology Letters 9:767–773.
Mittelbach, G. G., C. F. Steiner, S. M. Scheiner, K. L. Gross, H. L. Reynolds, R. B. Waide, M. R. Willig, S. I. Dodson, and L. Gough. 2001. What Is the Observed Relationship between Species Richness and Productivity? Ecology 82:2381–2396.
Molino, J.-F., and D. Sabatier. 2001. Tree Diversity in Tropical Rain Forests: A Validation of the Intermediate Disturbance Hypothesis. Science 294:1702–1704.
Moore, D. R. J., and P. A. Keddy. 1988. The Relationship between Species Richness and Standing Crop in Wetlands: The Importance of Scale. Vegetatio 79:99–106.
Oksanen, J. 1996. Is the Humped Relationship between species Richness and Biomass an Artefact due to Plot Size? Journal of Ecology 84:293–295.
Pierce, S., G. Brusa, I. Vagge, and B. E. L. Cerabolini. 2013. Allocating CSR plant functional types: the use of leaf economics and size traits to classify woody and herbaceous vascular plants. Functional Ecology 27:1002–1010.
Pierce, S., D. Negreiros, B. E. L. Cerabolini, J. Kattge, S. Díaz, M. Kleyer, B. Shipley, S. J. Wright, N. A. Soudzilovskaia, V. G. Onipchenko, P. M. van Bodegom, C. Frenette-Dussault, E. Weiher, B. X. Pinho, J. H. C. Cornelissen, J. P. Grime, K. Thompson, R. Hunt, P. J. Wilson, G. Buffa, O. C. Nyakunga, P. B. Reich, M. Caccianiga, F. Mangili, R. M. Ceriani, A. Luzzaro, G. Brusa, A. Siefert, N. P. U. Barbosa, F. S. Chapin III, W. K. Cornwell, J. Fang, G. W. Fernandes, E. Garnier, S. Le Stradic, J. Peñuelas, F. P. L. Melo, A. Slaviero, M. Tabarelli, and D. Tampucci. 2017. A global method for calculating plant CSR ecological strategies applied across biomes world-wide. Functional Ecology 31:444–457.
Pither, J., L. H. Fraser, A. Jentsch, M. Sternberg, M. Zobel, J. Cahill, C. Beierkuhnlein, S. Bartha, J. A. Bennett, B. Boldgiv, L. R. Brown, M. Cabido, G. Campetella, C. N. Carlyle, S. Chelli, A. M. Csergő, S. Diaz, L. Enrico, D. Ensing, A. Fidelis, H. W. Garris, H. A. L. Henry, M. Höhn, J. Klironomos, K. Koorem, R. Lawrence-Lodge, P. Manning, R. J. Mitchell, M. Moora, V. D. Pillar, G. C. Stotz, S. Sugiyama, S. Szentes, R. Tungalag, S. Undrakhbold, C. Wellstein, and T. Zupo. 2016. Response to Comment on “Worldwide evidence of a unimodal relationship between productivity and plant species richness.” Science 351:457–457.
Rapson, G. L., K. Thompson, and J. G. Hodgson. 1997. The Humped Relationship Between Species Richness and Biomass -- Testing its Sensitivity to Sample Quadrat Size. Journal of Ecology 85:99–100.
Shea, K., S. H. Roxburgh, and E. S. J. Rauschert. 2004. Moving from pattern to process: coexistence mechanisms under intermediate disturbance regimes. Ecology Letters 7:491–508.
Shipley, B., M. J. Lechowicz, I. Wright, and P. B. Reich. 2006. Fundamental Trade-Offs Generating the Worldwide Leaf Economics Spectrum. Ecology 87:535–541.
Tredennick, A. T., P. B. Adler, J. B. Grace, W. S. Harpole, E. T. Borer, E. W. Seabloom, T. M. Anderson, J. D. Bakker, L. A. Biederman, C. S. Brown, Y. M. Buckley, C. Chu, S. L. Collins, M. J. Crawley, P. A. Fay, J. Firn, D. S. Gruner, N. Hagenah, Y. Hautier, A. Hector, H. Hillebrand, K. Kirkman, J. M. H. Knops, R. Laungani, E. M. Lind, A. S. MacDougall, R. L. McCulley, C. E. Mitchell, J. L. Moore, J. W. Morgan, J. L. Orrock, P. L. Peri, S. M. Prober, A. C. Risch, M. Schütz, K. L. Speziale, R. J. Standish, L. L. Sullivan, G. M. Wardle, R. J. Williams, and L. H. Yang. 2016. Comment on “Worldwide evidence of a unimodal relationship between productivity and plant species richness.” Science 351:457–457.
VanDerZanden, A. M., and T. W. Cook. 2010. Sustainable Landscape Management: Design, Construction, and Maintenance. John Wiley & Sons.
Vellend, M. 2010. Conceptual Synthesis in Community Ecology. The Quarterly Review of Biology 85:183–206.
Venterink, H. O., M. J. Wassen, J. D. M. Belgers, and J. T. A. Verhoeven. 2001. Control of Environmental Variables on Species Density in Fens and Meadows: Importance of Direct Effects and Effects through Community Biomass. Journal of Ecology 89:1033–1040.
Wright, I. J., P. B. Reich, M. Westoby, D. D. Ackerly, Z. Baruch, F. Bongers, J. Cavender-Bares, T. Chapin, J. H. C. Cornelissen, M. Diemer, J. Flexas, E. Garnier, P. K. Groom, J. Gulias, K. Hikosaka, B. B. Lamont, T. Lee, W. Lee, C. Lusk, and J. J. Midgley. 2004. The worldwide leaf economics spectrum. Nature 428:821–827.
Xiao, S., R. Michalet, G. Wang, and S.-Y. Chen. 2009. The Interplay between Species’ Positive and Negative Interactions Shapes the Community Biomass-Species Richness Relationship. Oikos 118:1343–1348.